Key Words: European EHR Exchange Format, HL7 FHIR, X-Bundle, Digital Consumer Health Products, Quality Criteria, Quality Labelling.
This blog summarises the initial tangible outcomes accomplished by XpanDH’s Work Package 2 and outlines the next steps:
- A framework proposal for the quality labelling of Digital Consumer Health Products.
- An open repository of artefacts supporting future European EHR Exchange Format specifications, organized per adoption domain, including an ecosystem of HL7 FHIR (Fast Healthcare Interoperability Resources) Implementation Guides.
These achievements align with the declared objectives, as illustrated in Figure 1.

Figure 1 – XpanDH Work Package 2 Objectives
Quality labelling framework
To pave the way towards quality assurance of Electronic Health Record (EHR) and Digital Consumer Health Products (DCHP) a first framework version for quality labelling of Digital Consumer Health Products has been published.
The proposed framework aims to assist manufacturers in enhancing and efficiently demonstrating the quality of DHCPS. This, in turn, enables consumers and healthcare professionals to make informed decisions. Additionally, the framework provides valuable insights for insurers to make reimbursement decisions on DHCPs.
The approach follows a three-layered structure, progressively exploring the application of quality labelling. It begins at a generic level (DCHP), advances to the domain level (DCHP and EHR systems), and culminates in a detailed use-case-specific level (DCHP and Laboratory results).
The quality labelling framework has been drafted, drawing inspiration from existing standards, such as the 82304-2 Technical Specification concerning the quality and reliability of health and wellness apps, wellness apps, and labelling, as well as ISO 13131, ISO 10377, and ISO 13485. It also incorporates elements from previous European Commission projects, including Label2Enable, X-eHealth, and DigitalHealthEurope.
The proposed framework aims to offer comprehensive guidelines for use in the actual assessment of quality criteria, as depicted in Figure 2. The rationale for the general quality criteria framework for DCHPs is influenced by CEN-ISO 82304-2 Quality and reliability of health and wellness apps and activities conducted in the Label2Enable project. However, the framework’s criteria and questions specifically pertain to DCHPs, thereby, to a certain extent, extending the scope of TS 82304-2 to cover DCHPs as well.
The quality requirement questions, for uniformity with CEN-ISO TS 82304-2, have been grouped under six sections, with ‘Product information’ and five aspects of quality:
- Healthy and safe;
- Easy to use;
- Secure data;
- Robust build
- Continuous improvement
Identifying ‘consent and transparency’, ‘accessibility of the information’ and ‘portability, as the already outlined quality criteria to be further refined and elaborated for the next framework version.
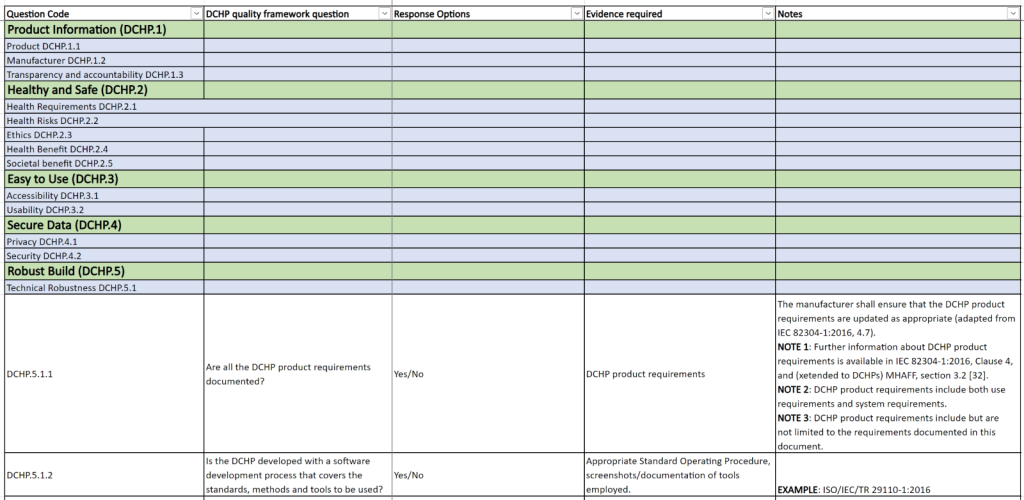
Figure 2 – Quality criteria for Digital Consumer Health Products
The HL7 FHIR Implementation Guide ecosystem
A single-entry point for the X-Bundle assets has been created to access a system of open GitHub repositories and HL7 FHIR Implementation Guides (IG). (the most updated version of this repository can be found at https://build.fhir.org/ig/hl7-eu/xpandh/).
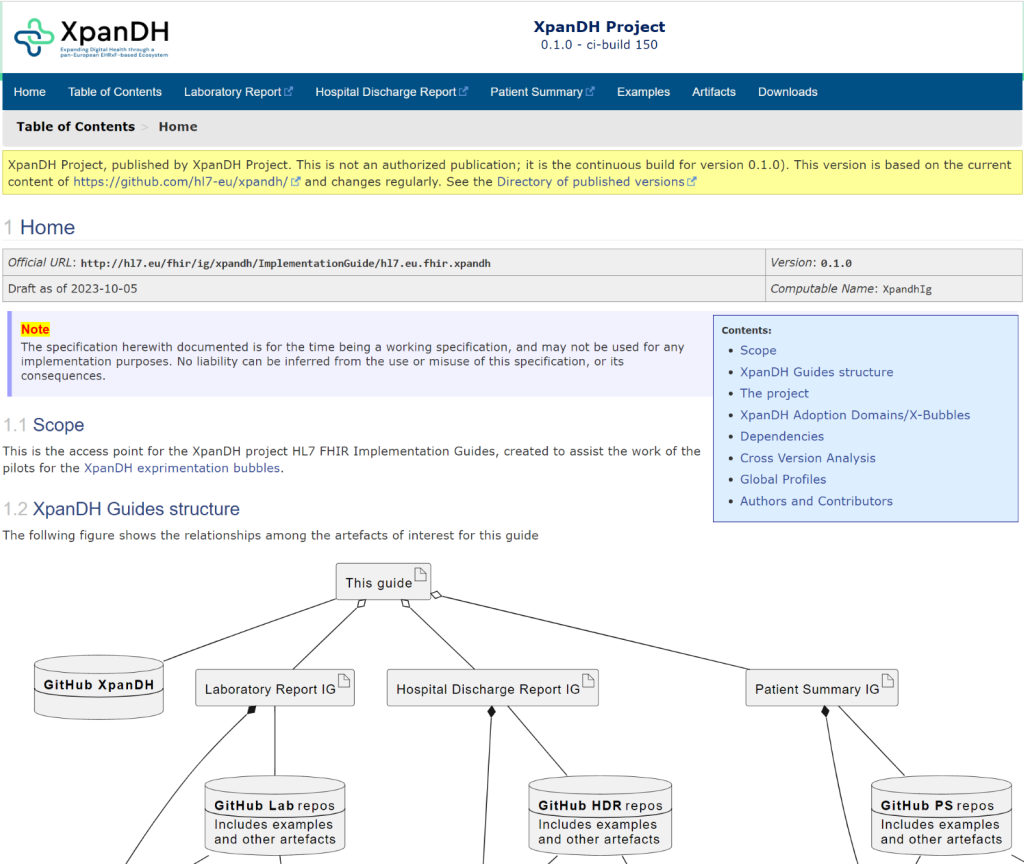
Figure 3 – XpanDH assets entry point (from D2.2)
A coherent ecosystem of technical specifications, in the form of HL7 FHIR IG, covering different priority domains (e.g., Laboratory, Hospital Discharge Report, Patient Summary) has been designed; and collaborations with relevant stakeholders established.
Thanks to these collaborations a standardized European Laboratory Report HL7 FHIR specification has been consolidated (see https://hl7.eu/fhir/laboratory/) as a result of a multi-stakeholder effort involving several European countries, projects and initiatives (e.g. MyHealth@EU).
A Public Comment session, ending in November 2023, has been arranged and received comments are under reconciliation. A dedicated track is being organized at the next January 2024 HL7 FHIR Connectathon in Athens, aiming to test the proposed specifications and identify potential adoption issues and gaps with existing implementations.
Moreover, the work of X-eHealth on Hospital Discharge Report FHIR specification has been continued (https://build.fhir.org/ig/hl7-eu/xpandh-hdr) and a first European Patient Summary HL7 FHIR representation proposed (https://build.fhir.org/ig/hl7-eu/xpandh-ps/).
In parallel with the specification work, WP2 is supporting the other XpanDH work packages in the adoption of these specifications to advance with the feedback collection.
What’s next
Quality labelling framework
A second version of the framework will be produced by the end of the project. This new version will refine and provide detailed quality criteria related to interaction and interoperability requirements that align with or should be supported by the infrastructure prescribed by the EEHRxF.
After the finalization of XpanDH, the same level of completeness of the 82304-2 TS should be reached by following a similar standardization path as adopted for that Technical standard.
The HL7 FHIR Implementation Guide ecosystem
The HL7 FHIR Implementation Guides (IGs) ecosystem will be expanded, beginning with new priority domains, likely starting with ePrescription, eDispensation, and Imaging Report.
The collaboration with users will continue to provide support in adopting HL7 FHIR specifications and collect examples and lessons learned.
Other feedback and improvements are expected to be reached during the in-person hands-on FHIR development and testing event organized in January as part of the HL7 Europe Working Group Meeting; where the proposed specification was further exercised.
About the author:
Giorgio Cangioli, Technical Leader at HL7 Europe
